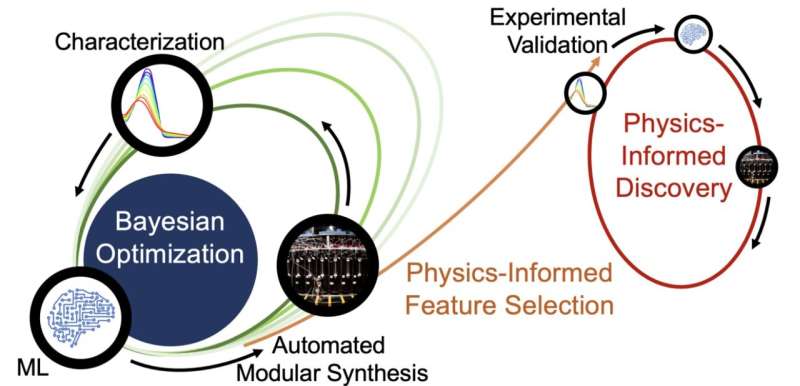
Closed-loop transfer paradigm for physics-based functional molecule discovery. Image credit: Nicholas Angello et al
Artificial intelligence is a powerful tool for researchers, but it has a significant limitation: it is impossible to explain how it arrived at its decisions. This problem is called the “AI black box.”
By combining AI with automated chemical synthesis and experimental validation, an interdisciplinary team of researchers at the University of Illinois Urbana-Champaign opened the black box and discovered the chemical principles that the AI relied on to improve molecules for solar energy harvesting.
The result was light-harvesting molecules four times more stable than the starting point and provided important new insights into what makes them stable – a chemical question that has hampered materials development so far.
The interdisciplinary research team was co-led by Martin Burke, professor of chemistry at the University of Toronto, Ying Diao, professor of chemical and biomolecular engineering, Nicholas Jackson, professor of chemistry and materials science and engineering, Charles Schroeder, in collaboration with Alán Aspuru-Guzik, professor of chemistry at the University of Toronto. They published their results in the journal Nature.
“New AI tools have incredible power. But when you try to look behind the scenes and understand what they’re doing, you usually come back with nothing useful,” Jackson said.
“For chemistry, this can be very frustrating. AI can help us optimize a molecule, but it can’t tell us why that’s the optimum – what are the important properties, structures and functions? Through our process, we’ve figured out what gives these molecules greater photostability. We’ve turned the AI black box into a transparent glass sphere.”
The researchers were motivated by the question of how to improve organic solar cells based on thin, flexible materials, as opposed to the rigid, heavy silicon-based panels that adorn roofs and fields today.
“What is hindering the commercialization of organic photovoltaics are stability issues. High-performance materials degrade when exposed to light, which is not what you want in a solar cell,” Diao said. “They can be manufactured and installed in ways that silicon cannot, and can also convert heat and infrared light into energy, but stability has been a problem since the 1980s.”
The Illinois method, called “closed-loop transfer,” begins with an AI-driven optimization protocol called a closed-loop experiment. The researchers asked the AI to optimize the photostability of light-harvesting molecules, Schroeder said.
The AI algorithm made suggestions about what types of chemicals to synthesize and study in multiple rounds of closed-loop synthesis and experimental characterization. After each round, the new data was integrated back into the model, which then provided improved suggestions, with each round getting closer to the desired outcome.
The researchers produced 30 new chemical candidates in five rounds of closed-loop experimentation, thanks to building block chemistry and automated synthesis developed by Burke’s group. The work was carried out in the Molecule Maker Lab of the Beckman Institute for Advanced Science and Technology at the University of Illinois.
“The modular chemistry approach beautifully complements the closed-loop experiment. The AI algorithm requests new data with maximum learning potential, and the automated molecule synthesis platform can generate the new required compounds very quickly. These compounds are then tested, the data flows back into the model, and the model gets smarter and smarter – over and over again,” said Burke, who is also a professor at Carle Illinois College of Medicine.
“So far, we have mainly focused on the structure. With our automated modular synthesis, we can now also explore the function.”
Furthermore, rather than simply ending the query with the final products picked out by the AI, as is the case with a typical AI-led campaign, the closed-loop transfer process aimed to uncover the hidden rules that made the new molecules more stable.
While the closed-loop experiment was running, another set of algorithms continuously monitored the resulting molecules and developed models of chemical properties that predict stability in the light, Jackson said. After the experiment was complete, the models provided new hypotheses that could be tested in the lab.
“We use AI to generate hypotheses that we can validate and then drive new human-led discovery campaigns,” Jackson said.
“Now that we have some physical descriptions of what makes molecules photostable, the screening process for new chemical candidates is much easier than searching blindly in chemical space.”
To test their photostability hypothesis, the researchers studied three structurally different light-harvesting molecules with the chemical property they identified – a specific high-energy region – and confirmed that choosing the right solvents made the molecules up to four times more photostable.
“This is a proof of concept. We are confident that we can tackle other material systems as well, and the possibilities are only limited by our imagination. Ultimately, we envision an interface where researchers can input a desired chemical function and the AI will generate hypotheses to test,” said Schroeder.
“This work could only be done with a multidisciplinary team and the people, resources and facilities we have in Illinois and at our collaborator in Toronto. Five groups have joined forces to produce new scientific insights that would not have been possible with the work of each subteam on its own.”
Further information:
Nicholas Angello et al., Closed-loop transfer enables artificial intelligence to gain chemical knowledge, Nature (2024). DOI: 10.1038/s41586-024-07892-1. www.nature.com/articles/s41586-024-07892-1
Provided by the University of Illinois at Urbana-Champaign
Quote: Team breaks through AI black box to find key chemistry for solar energy and beyond (August 28, 2024), accessed August 28, 2024 from https://phys.org/news/2024-08-ai-black-team-key-chemistry.html
This document is subject to copyright. Except for the purposes of private study or research, no part of it may be reproduced without written permission. The contents are for information purposes only.

